Optimized CLARITY procedures for faster, simpler and affordable whole brain clearing and imaging
The three key features of this new approach were: 1) accelerated clarification through parallelized flow-assisted clearing crucial for large cohorts independent of specialized equipment such as electrophoresis or perfusion chambers; 2) >90% cost reduction (also important for these large behavioral cohorts) using a new refractive index-matching process; and 3) optical properties such that the whole mouse brain can be imaged using a commercial light-sheet microscope (LSM) under a single field of view (FOV) and as a single stack (~1200 steps across a ~6.6mm range) in less than 2 hours with single-cell resolution throughout the whole volume (this speed and simplicity is also critical for large behavioral cohorts). Raw data files from each brain are ~12 GB in size and can be easily stored and directly analyzed on standard desktop workstations without the need for compression or stitching.
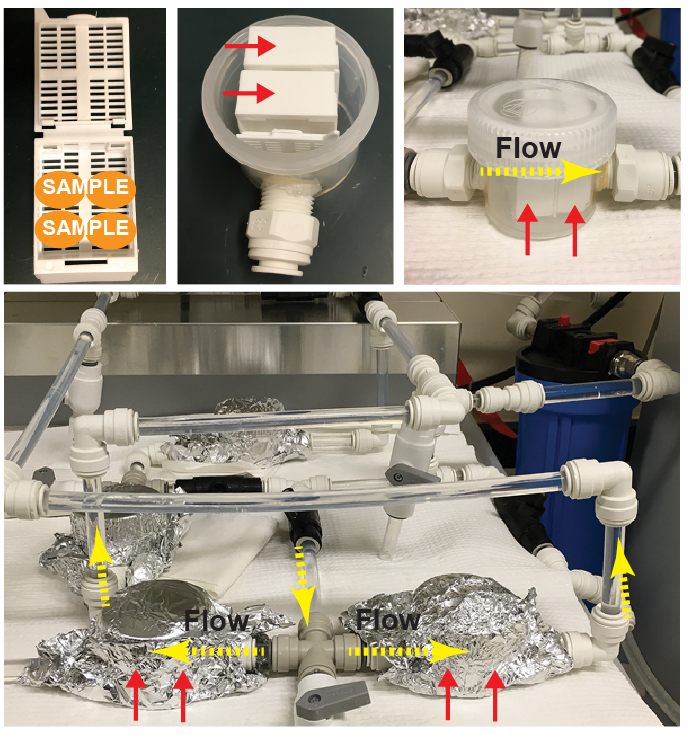
Setup of parallel flow-assisted clearing. Up to 4 mouse brains can be inserted into a tissue cassette (30x40x12mm). Two cassettes (indicated by red arrows) are inserted into a chamber constructed with an inlet and outlet for buffer exchange. To scale up clearing, multiple chambers (each containing up to 8 brains) can be connected in parallel to a temperature-controlled circulator (calibrated so that the temperature in the sample chamber is kept at 40°C).
A hydrogel based on 1% acrylamide (1% acrylamide, 0.0125% Bis, 4% PFA, 0.25% VA-044 initiator (w/v), in 1X PBS, as described in Tomer et al 2014) was used for all CLARITY preparations. Mice were transcardially perfused with ice-cold 4% PFA (not hydrogel yet). After perfusion, brains were post-fixed in 4% PFA overnight at 4°C and then transferred to 1% hydrogel for at least 48 hours to allow monomer diffusion. The samples were degassed and polymerized (4-5 hours at 37°C) in a 50ml tube. The brains were removed from hydrogel and washed with 200mM NaOH-Boric buffer (pH=8.5) containing 8% SDS for 6-12 hours to remove residual PFA and monomers. Brains could now be transferred to a flow-assisted clearing device using a temperature-control circulator or a simper combination of 50ml tube and heated stirring plate. 100mM Tris-Boric Buffer (pH=8.5) containing 8% SDS was used to accelerate the clearing (at 40°C). Note that Tris-containing buffer should only be used after PFA is completely washed out. With this setup, a whole mouse brain can be cleared in 12 days (with circulator, or 8 days for a hemisphere) or 16 days (with conical tube/stir bar). After clearing, the brain was washed in PBST (0.2% Triton-X100) for at least 24 hours at 37°C to remove residual SDS. Brains were incubated in a refractive index matching solution (RapidClear, RI=1.45, Sunjin lab, http://www.sunjinlab.com/) for 8-12 hours at 37°C and then 6-8 hours at room temperature. Sample can be stored in RC for several days at room temperature without affecting imaging quality. After the RC incubation, the brains were ready for imaging.
Alternative flow-assisted clearing setup without using a circulator. A 50ml conical tube (with small holes drilled in the middle and on the bottom, as indicated by red arrows) can be inserted into a 250ml glass bottle filled with clearing buffer. Each tube fits 3-4 mouse brains. Unidirectional flow (blue line) is created by using a magnetic stir bar and a stirring hot plate to accelerate the clearing. Upon first use, the temperature of the hot plate needs to be set properly so that the buffer temperature is maintained at desired level inside the conical tube.